Gaseous Elements and Noble Gases
Critical gases for modern industry and clean technology
The Invisible Inputs Behind Advanced Technologies
The gaseous elements represent a distinct and essential subset of the periodic table, encompassing both the inert noble gases and the highly reactive diatomic gases. These elements, such as helium, nitrogen, oxygen, fluorine, and chlorine, are foundational to life, industry, and the atmosphere. Noble gases are chemically stable and widely used in lighting, cryogenics, and semiconductor manufacturing, while diatomic gases play vital roles in combustion, respiration, and chemical synthesis. Despite their ubiquity, the production, storage, and strategic use of elemental gases present unique challenges due to their volatility, rarity (in the case of noble gases), and environmental implications. Some solid elements, such as iodine and bromine, also enter the gaseous phase under specific conditions, adding further complexity to this group. As technologies evolve and demand grows, especially in aerospace, electronics, medical imaging, and energy applications, the role of gaseous elements becomes increasingly critical. Explore the properties, classifications, and strategic uses of elemental gases below.
Noble gases
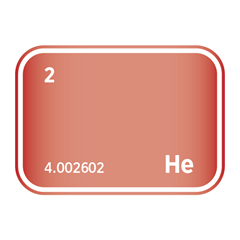
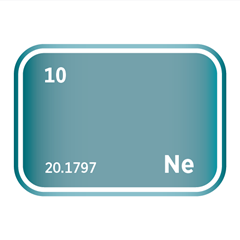
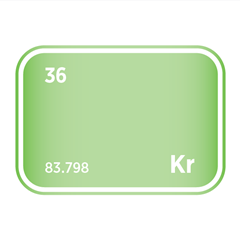
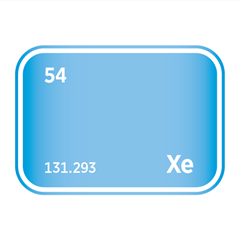
Noble gases, occupying Group 18 of the periodic table, are a unique family of elements defined by their exceptional chemical stability and low reactivity. This group includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Their defining feature is a complete outer electron shell, which renders them largely inert under standard conditions. As monoatomic gases, they exist naturally as isolated atoms rather than bonded molecules, and they exhibit consistent physical properties such as low boiling points and high ionisation energies.
Despite their limited reactivity, noble gases are far from industrially irrelevant. Helium is critical in cryogenics and superconducting technologies; neon and argon are essential in lighting, electronics, and semiconductor manufacturing, particularly in plasma etching and lithography processes. Krypton and xenon are used in ion propulsion, advanced lighting, and medical imaging applications. Several noble gases are also classified as high-purity electronic gases, required for the precise conditions demanded in chip fabrication and other microelectronic processes. Their scarcity, especially for helium and xenon, presents unique challenges for extraction and supply, making them strategically important in both advanced technologies and global resource management.
Non-metallic Diatomic Gases (at STP)
Non-metallic diatomic gases are a small but crucial subset of the periodic table, consisting of elements that exist naturally as two-atom molecules under standard temperature and pressure. This group includes hydrogen (H₂), nitrogen (N₂), oxygen (O₂), fluorine (F₂), and chlorine (Cl₂). Each of these elements forms stable covalent bonds with itself, resulting in diatomic molecules that are more energetically favourable than isolated atoms.
Standard temperature and pressure, or STP, refers to a defined set of conditions commonly used in chemistry to consistently compare gas behaviours. It is typically set at 0 degrees Celsius and 1 atmosphere of pressure. Under these conditions, the physical properties of gases, such as volume, density, and reactivity, can be accurately compared across elements.
These diatomic gases play fundamental roles across both natural and industrial systems. Oxygen and hydrogen are essential for combustion, energy production, and biological respiration. Nitrogen, making up most of Earth’s atmosphere, is widely used in agriculture, cryogenics, and electronics manufacturing. Fluorine and chlorine, though highly reactive, are critical feedstocks in chemical synthesis, pharmaceuticals, and industrial halogenation processes. The widespread utility of these gases, combined with their varied reactivity and environmental impact, underlines their strategic importance in global supply chains and modern technology.


Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Dr Jenny Watts
Critical Minerals Technologies Expert

Ismet Soyocak
ESG & Critical Minerals Lead

Thomas Shann Mills
Senior Machine Learning Engineer

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

Franklin Avery
Commodity Analyst

Shunjie Zhao (Tony)
Commodity Analyst: APAC

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.