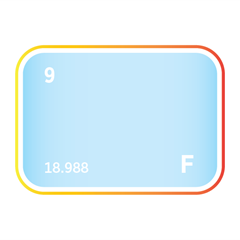
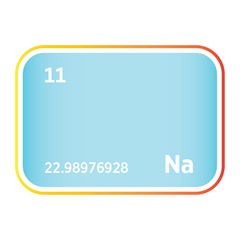
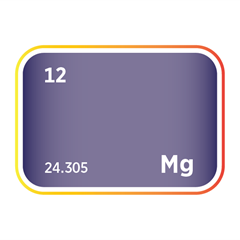
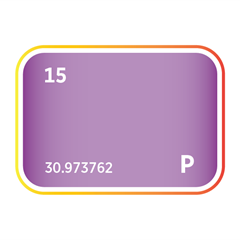
Bromine
Critical Minerals and The Energy Transition
Navigating the Bromine Market
Though less prominent than many industrial elements, Bromine is a strategically vital resource with a growing role in global supply chains. Its distinctive chemical reactivity enables a broad array of applications, spanning flame retardants, pharmaceuticals, water treatment, oil and gas drilling fluids, and high-tech batteries. As industries increasingly prioritise safety, energy storage, and environmental performance, bromine’s versatility and efficacy have brought it renewed strategic attention. In particular, bromine compounds are essential in fire safety regulations, and they are used extensively in plastics, electronics, and building materials to meet stringent flammability standards. The element also plays a critical role in zinc–bromine flow batteries, offering a scalable solution for stationary energy storage, which is vital for the integration of intermittent renewable energy sources. Its use in oilfield brines supports deep-well drilling and hydraulic fracturing, underpinning fossil fuel production in challenging environments. Global bromine supply is highly concentrated, with the United States, China, and Israel dominating production, often through brine extraction from salt lakes or underground reservoirs. This geographic concentration creates potential vulnerabilities in supply resilience, especially as demand intensifies across clean energy and defence-linked applications. Regulatory pressures, environmental concerns, and water resource constraints may also affect future production capacity. As energy systems evolve and industrial safety standards tighten, bromine’s significance continues to grow. SFA (Oxford) examines the complex supply, regulatory, and technological factors shaping the bromine market, highlighting its increasingly critical role in global decarbonisation strategies, industrial resilience, and clean technology innovation.
An introduction to bromine
Bromine demand and end-uses
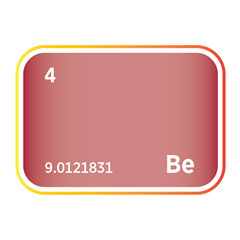
Bromine is a dense, red-brown, highly reactive halogen element with distinctive chemical properties that make it valuable across flame retardants, oil and gas drilling, water treatment, pharmaceuticals, and electronics. Though produced in moderate global volumes, bromine is essential in many high-value industrial applications because it can form stable organic and inorganic compounds. Its reactivity, volatility, and versatility ensure strong demand across established and emerging technologies.
The most extensive use of bromine is in producing brominated flame retardants (BFRs), which are added to plastics, textiles, electronics, and construction materials to reduce flammability and suppress combustion. Brominated compounds interfere with the chemical reactions in fire propagation, effectively limiting ignition and slowing fire spread. BFRs are widely used in electronics housings, circuit boards, insulation materials, and automotive components, particularly in applications where fire safety standards are stringent. While some legacy brominated flame retardants have been phased out due to environmental concerns, newer formulations with improved ecological profiles continue to drive demand, especially in the electrical and transport sectors.
In the oil and gas industry, bromine produces clear brine fluids, particularly zinc bromide and calcium bromide, which are employed in well completion and workover operations. These high-density fluids help control pressure in high-temperature, high-pressure wells and prevent blowouts while preserving well integrity. Bromine-based brines are also used in drilling and fracturing operations, especially in offshore and deep-well projects where operational safety and fluid performance are paramount.
Bromine is critical in water treatment and sanitation, particularly in industrial cooling systems, swimming pools, and hot tubs, where it is used as an alternative to chlorine. Bromine is more stable at high temperatures and across a wider pH range, making it suitable for disinfection in challenging water conditions. Brominated compounds such as bromochlorodimethylhydantoin (BCDMH) release bromine slowly and consistently, providing long-lasting antimicrobial control against bacteria, algae, and fungi.
In the pharmaceutical and agrochemical industries, bromine is used as a synthesis intermediate, producing sedatives, antihistamines, and antiepileptic drugs. Brominated compounds are also used in fungicides, herbicides, and insecticides, offering targeted pest and disease control. While bromine-based methyl bromide was once widely used as a soil fumigant, it has been largely phased out due to ozone depletion concerns under the Montreal Protocol.
Bromine is used to produce dyes, photographic chemicals, and organic intermediates, particularly where halogenation reactions are required. It is also employed in analytical chemistry, mercury emission control, and electronic-grade processes, including in lithium-ion battery electrolyte additives to improve stability and cycle life.
In energy storage, bromine is used in zinc–bromine and hydrogen–bromine flow batteries, where its reversible redox chemistry and high energy density support long-duration storage solutions. These battery systems are being explored for grid-scale applications, particularly in regions seeking alternatives to lithium-based chemistries for stationary energy storage.
Bromine demand is expected to grow steadily, supported by rising requirements in flame retardants, high-performance drilling fluids, energy storage systems, and water treatment technologies. While some environmental and regulatory pressures exist, particularly concerning specific brominated compounds, newer, more environmentally benign formulations and strategic applications continue to underpin long-term demand. Innovation in bromine-based batteries and mercury control technologies may open new growth areas aligned with clean energy and emissions reduction goals.
With its unique chemistry and irreplaceable role in fire safety, sanitation, energy, and synthesis, bromine remains a critical element in modern industry. Its balance of reactivity, stability, and compatibility with other halogens ensures continued relevance across both legacy and next-generation technologies. As resource efficiency and regulatory oversight improve, bromine’s strategic role in supporting resilient infrastructure, clean technology, and public safety is set to expand.

Bromine supply
Global bromine production is highly concentrated, with the majority of output originating from a handful of countries, most notably Israel, the United States (particularly Arkansas), China, and Jordan. These regions host rich bromine-bearing brine deposits that are economically viable to extract, often located in geological settings associated with ancient evaporite basins. Bromine is typically recovered via solar evaporation or chemical extraction from subterranean brines, rather than mined in solid form.
Bromine is not found in elemental form in nature but is present in seawater and brine sources, often alongside other critical elements such as lithium, magnesium, and potassium. While seawater contains bromine, its low concentration makes direct extraction economically unfeasible, so commercial operations focus on high-grade brines. Co-occurring elements can influence processing economics, with integrated extraction strategies sometimes improving resource efficiency.
Israel remains a global leader in bromine production, with extensive operations in the Dead Sea region operated by ICL Group. The high bromine content of the Dead Sea brines, coupled with favourable climate conditions for solar evaporation, has enabled Israel to maintain high-purity, large-scale output for decades. Similarly, in the United States, Albemarle Corporation extracts bromine from deep brine wells in southern Arkansas, which represents the country's only major production zone. The U.S. supply chain benefits from advanced infrastructure and well-developed downstream chemical industries.
China also contributes significantly to global bromine supply, mainly through inland brine deposits in provinces such as Shandong. However, Chinese production is typically more fragmented and environmentally constrained, with increased regulatory pressure on brine extraction and chemical waste management. Jordan’s bromine production is also based around the Dead Sea and is operated in conjunction with its potash industry, contributing to regional supply diversification in the Middle East.
In addition to these leading producers, other bromine-supplying nations include Japan, Ukraine, India, Germany, Turkmenistan, and Russia. While their output is comparatively smaller or less commercially visible, they contribute to niche markets and regional demand, and in some cases, maintain strategic reserves or integrated chemical production capacity. These secondary producers add a layer of supply resilience, though the market remains dominated by a concentrated core of high-output operations.
Bromine is often co-produced alongside other industrial minerals, notably magnesium, lithium, potash, and sodium compounds. This interdependence creates both opportunities and trade-offs. For example, in the Dead Sea and specific Chinese brine fields, bromine extraction is integrated into broader operations targeting potash and magnesium. While this can reduce per-unit extraction costs and optimise brine resource use, it also ties bromine supply to adjacent mineral sectors' market conditions and operational decisions. For instance, a downturn in potash demand or operational disruptions in lithium production may inadvertently constrain bromine output, even if bromine demand remains strong.
Supply dynamics are shaped not only by the distribution of bromine-rich brines but also by access to water rights, energy availability for evaporation or chemical processing, and proximity to downstream chemical markets. Environmental concerns regarding the disposal of brominated waste and brine reinjection practices are increasingly influencing production practices and regulatory compliance costs.
Environmental scrutiny of bromine production is intensifying, particularly around brine disposal, emissions from brominated compounds, and the impact of halogenated waste streams. In China, for instance, tighter enforcement of environmental standards has led to the temporary suspension of smaller bromine operations, affecting domestic supply and export volumes. In the U.S., concerns over groundwater contamination and reinjection practices influence permitting processes for expansion. These trends suggest that regulatory and ESG constraints may limit supply growth without significant investment in cleaner production technologies, even where reserves are ample.
Bromine’s geographic concentration and reliance on a few producers pose strategic vulnerabilities. The dominance of Israel and the U.S. in bromine exports leaves markets exposed to regional disruptions, from water allocation challenges in the Dead Sea basin to regulatory shifts in U.S. environmental policy. Moreover, increasing geopolitical frictions between China and the West could complicate the flow of brominated intermediates used in flame retardants, pharmaceuticals, and agrochemicals. Countries lacking domestic bromine resources may face procurement risks in sensitive industries if global trade in halogenated compounds becomes more tightly controlled or subjected to export restrictions.
Recycling of bromine remains limited, mainly due to the dissipation of the element during use and the difficulty of recovery from complex end-of-life products. In flame retardants, for example, bromine is chemically bonded into polymers and dispersed widely in consumer goods, construction materials, and electronics. Once incinerated or discarded, bromine is often lost irretrievably. While growing interest is in developing closed-loop systems, particularly for high-value industrial applications like drilling fluids or advanced battery chemistries, economies of scale and technical feasibility still pose significant hurdles. As such, global bromine supply remains overwhelmingly reliant on virgin extraction.
Bromine producers
Please note that in the United States, primary producers sell or use elemental bromine to create various bromine compounds.
Bromine substitution
Substitution for bromine is highly application-specific and often constrained by performance, cost, and regulatory trade-offs. In many of its key uses, such as flame retardants, drilling fluids, and certain pharmaceutical intermediates, bromine offers a combination of chemical reactivity and thermal stability that is difficult to replicate with alternative materials.
In flame retardants, where brominated compounds have long been dominant, growing environmental and health concerns have driven the development of substitutes such as phosphorus-based, nitrogen-based, and mineral-based formulations (e.g. aluminium hydroxide or magnesium hydroxide). These alternatives are increasingly used in textiles, electronics, and construction materials, particularly in jurisdictions like the European Union, where restrictions on persistent organic pollutants have tightened. However, non-brominated flame retardants may require higher loading levels, compromise performance, or raise costs in high-specification applications, limiting their broader adoption.
In oil and gas drilling fluids, bromine is commonly used as calcium bromide or zinc bromide brines, valued for their high density and thermal stability in high-pressure, high-temperature wells. While alternatives like formate brines (based on potassium or sodium formate) and cesium formate exist, they are more expensive or less effective under extreme conditions. Substitution is therefore typically confined to wells with less demanding performance requirements.
For specific niche uses, such as photographic chemicals, disinfectants, and water treatment, chlorine- or iodine-based compounds can act as substitutes, but often at a loss of efficiency or selectivity. In the pharmaceutical sector, brominated intermediates are sometimes replaced with fluorinated or chlorinated analogues. Still, these alternatives can alter drug efficacy, synthesis pathways, or regulatory classification, meaning substitution is assessed case by case.
Overall, while viable substitutes for bromine exist in several industrial uses, their adoption is governed by stringent technical, economic, and regulatory constraints. As bromine faces increasing environmental scrutiny, demand may shift toward less persistent and bioaccumulative compounds. Still, in many high-performance applications, bromine remains difficult to displace without compromising end-product functionality.



Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Dr Jenny Watts
Critical Minerals Technologies Expert

Ismet Soyocak
ESG & Critical Minerals Lead

Thomas Shann Mills
Senior Machine Learning Engineer

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

Franklin Avery
Commodity Analyst

Shunjie Zhao (Tony)
Commodity Analyst: APAC

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.