Helium
Critical Minerals and The Energy Transition
Navigating the Helium Market
Helium, the second lightest element and a noble gas known for its low density, inertness, and the lowest boiling point of any element, is pivotal in various high-tech, scientific, and medical applications. Its unique properties make it indispensable for cooling superconducting magnets in MRI machines, providing lift in airships and balloons, and as a protective atmosphere for arc welding and semiconductor manufacturing. The helium market is characterised by its complex supply chain, rooted in a limited number of natural gas fields capable of producing commercially viable quantities of helium. This scarcity, combined with the growing demand across diverse sectors—from healthcare diagnostics to space exploration and fibre optics manufacturing—underscores the strategic importance of helium and the challenges in ensuring its availability. The global quest for sustainable helium sources has intensified, highlighting the need for innovative extraction methods, recycling, and conservation strategies to address the vulnerabilities of helium supply against the backdrop of its expanding applications. As the world leans more into technological advancements and scientific research, helium's role becomes increasingly critical, reflecting a broader narrative of balancing resource scarcity with the demands of progress and innovation.
An introduction to helium
Helium demand and end-uses
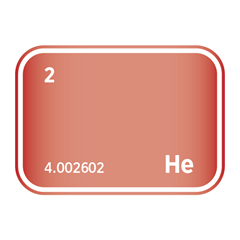
Helium is a colourless, odourless, inert gas with unique physical and chemical properties that make it irreplaceable in various advanced technologies, scientific research, and industrial processes. As the second lightest element and a noble gas, helium is non-reactive, has the lowest boiling point of any element, and exhibits exceptional thermal conductivity, making it essential where extreme conditions or precision are involved. Despite its abundance in the universe, helium is rare on Earth and is primarily recovered from natural gas fields, making its supply limited, geographically concentrated, and strategically significant.
One of helium’s most critical uses is in cryogenics, where it is the only element cold enough to liquefy and maintain temperatures near absolute zero. Liquid helium is essential for cooling superconducting magnets used in magnetic resonance imaging (MRI) machines, particle accelerators, nuclear magnetic resonance (NMR) spectroscopy, and quantum computing. The healthcare sector is the largest single consumer of liquid helium, and as MRI remains a cornerstone of diagnostic imaging, a consistent helium supply is vital to global medical infrastructure.
Helium is also essential in semiconductor manufacturing, where it is used as a process gas to cool components, maintain ultra-pure environments, and support plasma etching and deposition techniques. As device geometries shrink and fabrication becomes more complex, helium’s role in controlling heat, suppressing contamination, and stabilising production environments becomes increasingly essential. Advanced chip manufacturing facilities, especially for high-end logic and memory chips, are significant consumers of high-purity helium.
In aerospace and defence, helium is used for pressurising and purging rocket fuel tanks, cooling missile guidance systems, and as a carrier gas in wind tunnels and aerodynamics testing. Its inertness and low density make it ideal for applications where chemical stability and precision under pressure are essential. Helium has long played a role in space exploration, including in the launch and operation of crewed and uncrewed missions.
Helium is widely used in leak detection, where its small atomic size and inertness allow it to escape from minute cracks or imperfections in sealed systems. It is employed in quality assurance processes across automotive manufacturing, refrigeration, high-vacuum systems, and pharmaceuticals, where even tiny leaks can affect safety, performance, or sterility.
Helium is used as a shielding gas for arc welding of materials such as aluminium, stainless steel, and titanium. It helps produce clean, stable arcs and deeper weld penetration, particularly in applications that require high strength and precision, including aerospace components and high-performance equipment.
Helium also plays a vital role in lifting and buoyancy, most notably in weather balloons, airships, and scientific balloons. Unlike hydrogen, helium is non-flammable, making it a safer alternative for lighter-than-air applications. Although its use in party balloons has historically been widespread, this sector is declining due to conservation efforts and rising prices.
In scientific research, helium is used as a carrier gas in gas chromatography and mass spectrometry, where its inertness and low viscosity ensure high-resolution analysis. It is also used in nuclear energy research and cryo-laboratories, including superconductors, quantum materials, and low-temperature physics research.
Helium demand is expected to grow, particularly from sectors such as healthcare, semiconductors, quantum technologies, and space exploration. However, helium supply faces challenges due to the limited number of suitable production sites, geopolitical tensions, and the decline of some conventional gas fields. Supply risks have prompted new exploration in North America and Africa, increased interest in strategic reserves, and greater emphasis on recycling and conservation technologies, particularly MRI systems.
Helium’s unique physical properties and its irreplaceability in critical sectors ensure it remains a strategic element in science, medicine, and advanced manufacturing. From cooling superconductors to enabling deep space missions and producing cutting-edge semiconductors, helium supports innovation and reliability across technologies that define modern life and future progress.
Strategic applications of Helium
Helium supply
Helium is a non-renewable noble gas primarily extracted from natural gas fields with unusually high helium concentrations. It is not mined from the atmosphere, where it is present only in trace amounts, but instead produced during natural gas processing at fields that typically contain 0.1–2% helium by volume. Commercial recovery occurs through cryogenic distillation, a highly specialised and energy-intensive process that separates helium from other gases after processing natural gas. Because helium escapes into the atmosphere if not captured during this stage, it is fundamentally non-recoverable and considered a finite resource, only viable from specific geological formations.
Helium is formed over geological timescales through the radioactive decay of uranium and thorium deep within the Earth's crust. It accumulates in natural gas reservoirs where impermeable rock traps it alongside hydrocarbons. Economically recoverable helium is found in only a few locations worldwide, making its supply geologically constrained and geographically concentrated. The largest and most mature production zone is in the United States, particularly in the Hugoton-Panhandle complex spanning Texas, Oklahoma, and Kansas. Other major producers include Qatar, Algeria, and Russia, countries with significant natural gas infrastructure capable of cryogenic separation.
Helium’s unique physical properties, such as being chemically inert, the second lightest element, and the coldest cryogen, make it indispensable across strategic industries. However, these same properties mean it escapes Earth’s atmosphere once released, unlike other critical materials that can be recycled. Poor system management and venting practices continue to result in permanent helium loss, compounding long-term supply concerns.
Global helium supply has historically been dominated by a handful of producers and traders, leading to periodic shortages and sharp price fluctuations. The U.S. Federal Helium Reserve, which once stabilised the market by providing a buffer stock, is nearing the end of its operational life, creating uncertainty around future supply resilience. Spot markets have become increasingly volatile, particularly affecting high-purity users in medical imaging, semiconductor manufacturing, aerospace, and research sectors, who have faced rationing during global shortages.
Geopolitical events have further shaped helium’s supply profile. Russia’s Amur gas processing facility, once expected to become a primary new source, suffered a significant fire in 2021 and has faced continued setbacks amid international sanctions following the invasion of Ukraine. These disruptions have delayed expected volumes and increased supply risk, reinforcing concerns over dependence on a small number of politically exposed producers.
In response, new sources are being explored. Primary helium ventures in Tanzania's Rukwa Basin and Canada’s Saskatchewan Basin target reservoirs rich in helium but low in hydrocarbons. These efforts aim to diversify production and de-risk supply chains from broader fossil fuel market dynamics. However, such projects remain in early stages and face infrastructure and investment challenges.
Helium’s strategic importance continues to rise as demand expands in quantum computing, space technologies, and fibre optics, particularly across Asia. Countries like China are now investing in domestic extraction, storage, and conservation strategies, while others are reclassifying helium as a critical raw material to better safeguard future access. Conservation and recycling technologies, particularly for MRI and cryogenic systems, are gaining prominence as essential tools to improve efficiency and reduce losses. Together, these approaches reflect a shifting global recognition of helium’s irreplaceable role, and the urgent need to manage it sustainably.
Helium substitution
Substituting helium is inherently challenging due to its unparalleled physical and chemical properties. As an inert, low-density, and ultra-cold gas, helium serves critical functions that few other elements can perform without significant compromises in safety, efficiency, or cost. Substitution is typically limited to non-critical uses or supported by operational trade-offs.
There is no alternative to helium in cryogenic applications that require temperatures below −429 degrees Fahrenheit. Helium remains the only viable coolant for many superconducting systems used in magnetic resonance imaging (MRI), quantum computing, and particle physics. However, nitrogen is being explored as a cooling medium in emerging high-temperature superconductors. While this could reduce reliance on helium in specific applications, it does not eliminate the need for helium in systems that operate at temperatures near absolute zero.
Helium provides superior arc stability and heat penetration in welding, particularly for materials like aluminium and titanium in aerospace and high-specification industries. Argon can substitute helium in less demanding contexts, though it typically results in lower weld quality or slower performance. Helium’s small atomic size and inertness make it ideal for leak detection systems. Still, hydrogen and forming gas blends can sometimes serve as lower-cost substitutes, albeit with safety and sensitivity trade-offs.
Hydrogen is also being evaluated as an alternative to helium in deep-sea diving gas mixtures, where its properties support physiological stability under extreme pressures. However, flammability and handling risks remain significant barriers. Similarly, hydrogen can replace helium in buoyancy applications like balloons or blimps, where fire risk is acceptable, although it is rarely used due to historical safety concerns.
In less critical applications such as party balloons or novelty uses, helium substitution is straightforward—air or nitrogen are adequate and environmentally preferable. These efforts are part of broader conservation strategies to preserve helium for essential sectors such as medical imaging, aerospace, and semiconductor manufacturing.
Ultimately, substitution for helium depends on the specific functional demands of each application. While alternatives exist in some domains, none match helium’s unique combination of safety, inertness, and cryogenic capability. As supply constraints tighten, the focus will shift toward conservation technologies, closed-loop recovery systems, and selective substitution where feasible.



Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Ismet Soyocak
ESG & Critical Minerals Lead

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.