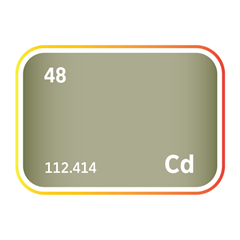
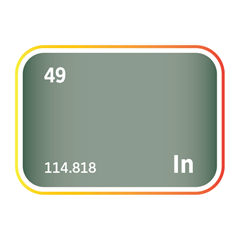
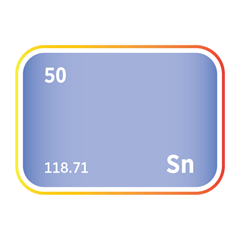
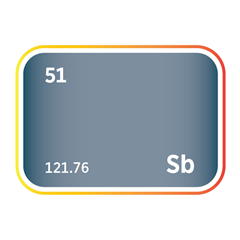
Mercury
Critical Minerals and The Energy Transition
Navigating the Mercury Market
Mercury, a unique metal known for its liquid state at room temperature and mesmerising silvery appearance, occupies a complex position within the global market. Historically revered for its various applications, from traditional medicine and dental amalgams to industrial processes, mercury's use today is heavily regulated due to its toxicity and the environmental and health risks associated with mercury exposure. The Minamata Convention on Mercury, a global treaty adopted to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds, reflects the international commitment to reducing mercury use and preventing its release into the atmosphere. Despite these challenges, mercury remains in demand for specific niche applications, such as manufacturing fluorescent lamps, certain types of chemical production, and artisanal small-scale gold mining, albeit under stringent controls. The mercury market is thus characterised by its dual need to satisfy ongoing but declining demand while advancing efforts to find safer alternatives and enhance mercury recovery and recycling practices. As the world moves towards more sustainable practices, the mercury market's future hinges on balancing these diminishing uses with global health and environmental sustainability goals, making the transition away from mercury a key focus for industries and policymakers alike.
An introduction to mercury
Mercury demand and end-uses
Mercury is a dense, silvery liquid metal known for its unique physical properties, including being the only metallic element that remains liquid at room temperature. While global use of mercury has declined significantly due to its toxicity and associated environmental and health risks, it still plays a role in several industrial, scientific, and artisanal applications. Mercury’s chemical reactivity, high density, and ability to form stable amalgams have historically made it valuable across diverse sectors. However, its use is now subject to strict regulation in most countries.
One of the most persistent areas of mercury demand is in artisanal and small-scale gold mining (ASGM), particularly in parts of Africa, South America, and Southeast Asia. Mercury extracts gold from ore by forming an amalgam, which is then heated to evaporate the mercury, leaving behind gold. This process is simple and accessible but poses serious health and environmental risks, particularly in communities with limited regulatory oversight. ASGM remains the most significant global source of intentional mercury use and a major contributor to atmospheric mercury emissions.
In the chemical industry, mercury has historically been used as a catalyst in producing chlorine and caustic soda through mercury-cell electrolysis. While most countries have phased out this method in favour of membrane or diaphragm technologies, some legacy capacity remains, particularly in developing economies. Mercury is also used in the production of vinyl chloride monomer in some regions, although this practice is also in decline due to international efforts to eliminate high-risk uses.
Mercury is used specifically in scientific instruments and laboratory equipment, particularly in older devices such as thermometers, barometers, manometers, and certain types of vacuum pumps. These applications exploit mercury’s predictable expansion with temperature, density, and low vapour pressure. While digital and alternative liquid-based devices have replaced mercury in many countries, some precision scientific uses continue, especially in niche research settings.
Mercury vapour is used in fluorescent, high-intensity discharge (HID), and cold cathode lamps. These lamps produce ultraviolet light by exciting mercury vapour, stimulating a phosphor coating to emit visible light. Despite the growing shift toward LED technology, mercury-based lamps are still used in industrial, commercial, and municipal lighting, particularly where colour rendering, intensity, or cost constraints limit alternatives.
Mercury is also used in electrical and electronic applications, such as tilt switches, relays, and rectifiers. These components rely on mercury’s conductive and liquid properties to operate under sealed conditions. However, such uses have largely been discontinued in favour of solid-state or mechanical alternatives, except in some specialised military or aerospace applications where reliability and durability in extreme environments are critical.
In dentistry, mercury is a dental amalgam component used for tooth fillings, composed of mercury mixed with silver, tin, and other metals. While mercury amalgams have been widely used for decades, many countries have restricted or banned their use due to concerns over mercury exposure and environmental release, especially from crematoria and wastewater systems. Alternatives such as resin-based composites are increasingly preferred.
Mercury has also been used in pharmaceuticals and cosmetics, particularly in preservatives like thimerosal and skin-lightening creams. However, due to toxicity concerns, these uses are now tightly controlled or banned in many jurisdictions. Thimerosal is still used in trace amounts in some vaccines as a preservative, although this is rare and declining.
Global mercury demand is expected to continue declining, driven by regulatory bans, substitution, and international agreements such as the Minamata Convention on Mercury, which aims to reduce anthropogenic mercury emissions and protect human health and the environment. However, demand from ASGM and some industrial processes remains persistent, highlighting the need for technical support, education, and accessible alternatives in affected regions.
While mercury’s legacy as a valuable but hazardous material is undeniable, its ongoing use presents environmental, social, and ethical challenges. Its future lies not in expansion but in responsible phase-out, improved waste management, and the deployment of safer substitutes. As global efforts intensify to eliminate mercury from industrial ecosystems, its strategic importance is being replaced by a coordinated effort to mitigate its impact and ensure a safer, more sustainable approach to industrial development.

Mercury supply
Most mercury is obtained as a by-product of non-ferrous metal mining, particularly from deposits of cinnabar (mercury sulphide) or extracted during refining gold, silver, zinc, or lead. Primary mercury mining is minimal, with most supply from recycling, recovery from waste streams, and stocks from decommissioned industrial facilities. China, Mexico, and Kyrgyzstan have historically been among the largest mercury producers, although international pressure under the Minamata Convention has curtailed primary production.
Mercury supply is highly constrained, geopolitically sensitive, and increasingly shaped by international environmental policy, with global production concentrated in just a few countries. Historically extracted as cinnabar (mercury sulphide), mercury is one of the few metals in liquid form at ambient temperature. However, due to its acute toxicity and long-term environmental persistence, mercury supply is now subject to strict regulation under the Minamata Convention on Mercury, which aims to phase out non-essential uses and limit emissions and trade.
Primary mercury production today is extremely limited and largely confined to Kyrgyzstan, which operates the Khaidarkan mine, one of the world’s last active mercury mines. Despite longstanding international pressure to cease operations, Kyrgyzstan has continued production due to economic dependence on the mine and a lack of viable alternatives for local employment. China, once the world’s largest mercury producer, has dramatically curtailed primary output in recent years in response to environmental policy and falling demand. Some by-product mercury is also recovered during gold mining and non-ferrous smelting operations in countries like Peru, Mexico, and Indonesia, where it is extracted from cinnabar-rich ores or sulphide mineral residues. However, these flows are often poorly tracked and operate within artisanal or small-scale sectors where mercury control remains weak.
The European Union, North America, and Japan have banned primary mercury mining and tightly restrict its use, with legal supply derived almost entirely from recycling or stockpiled material. Recycled mercury from end-of-life products such as thermometers, dental amalgam, fluorescent lamps, and industrial sensors is now the dominant source of legal mercury in many jurisdictions. However, the economics of mercury recycling are often unfavourable due to labour costs, health and safety requirements, and declining availability of mercury-containing products as phase-outs continue. Safe handling, containment, and long-term storage of surplus mercury are becoming a growing policy focus as countries seek to prevent re-entry into illicit trade channels.
Informal and illegal mercury supply chains, particularly those linked to artisanal and small-scale gold mining (ASGM), undermine formal supply controls. ASGM is the single largest source of anthropogenic mercury emissions globally, with mercury often smuggled into regions with lax enforcement, where it is used to amalgamate gold from ore. This illicit supply chain is difficult to quantify, highly decentralised, and frequently overlaps with broader issues of poverty, land tenure, and unregulated mining. Efforts by the United Nations and NGOs to introduce mercury-free gold extraction technologies and improve traceability have had mixed success, with uptake dependent on education, financing, and enforcement capacity.
International agreements have tightened the monitoring of mercury trade. Under the Minamata Convention, exports are banned for specific end uses, and prior informed consent is required for cross-border mercury shipments. Strategic mercury stockpiles maintained by countries such as the United States and Switzerland are subject to strict controls, and commercial trading is limited to approved industrial uses. As more nations ratify and implement the Convention, the scope of legal primary production and trade continues to shrink.
Despite these constraints, mercury remains essential for a small number of high-priority, technically challenging applications. These include nuclear instrumentation, calibration devices, scientific research, and some military systems with no suitable alternatives. Mercury’s unique physical properties—such as high density, electrical conductivity, and vapour pressure, also sustain niche demand in chlor-alkali production (where legacy mercury cell technology persists in a few plants), and in certain fluorescent and UV lamps still used in industrial and medical contexts. However, mercury-free alternatives are declining or replacing most of these uses.
The global mercury supply outlook is one of managed decline, with falling demand in most sectors, heightened regulatory scrutiny, and growing emphasis on recycling, containment, and substitution. Environmental and health concerns will continue to limit investment in new supply. At the same time, international efforts focus on phasing out mercury from legacy systems, curbing illegal ASGM-related use, and developing long-term disposal solutions for surplus material.
Current mercury producers
Mercury substitution
Substitution of mercury is widespread and driven by environmental regulation, health concerns, and international agreements such as the Minamata Convention, which aims to reduce mercury emissions and eliminate non-essential uses. Due to its acute toxicity, bioaccumulative nature, and persistence in ecosystems, mercury has been phased out or replaced in many applications across industry, medicine, and consumer products. Substitutes vary depending on the specific function mercury serves—be it conductivity, density, vapour pressure, or chemical reactivity—and the trade-offs involved in performance, cost, and sustainability.
In lighting, mercury has traditionally been used in fluorescent, high-intensity discharge (HID), and ultraviolet (UV) lamps, enabling efficient light generation through arc discharge. Substitution is now well advanced, with light-emitting diode (LED) and organic LED (OLED) technologies offering superior energy efficiency, longer lifespans, and mercury-free operation. LEDs have almost entirely displaced mercury in general lighting, signage, and display applications. However, mercury-containing UV lamps are still used in some industrial, disinfection, and medical settings where alternatives remain under development.
In dental applications, mercury amalgam was long used for cavity fillings due to its durability and ease of placement. Today, amalgam is increasingly replaced by composite resins, glass ionomer cements, and ceramic-based materials. These substitutes offer acceptable performance, improved aesthetics, and lower toxicity, though they may be less durable in high-load areas or more technique-sensitive during placement. Many countries have introduced restrictions or complete bans on mercury amalgam use, particularly for vulnerable populations such as children and pregnant women.
In chlor-alkali production, mercury-cell electrolysis has been a primary industrial process for generating chlorine and caustic soda. However, most facilities have now transitioned to membrane cell or diaphragm cell technologies, which are more energy efficient and eliminate mercury use. The phase-out of mercury cells is nearly complete in Europe and North America, with residual capacity concentrated in regions where capital constraints, regulatory gaps, or legacy infrastructure have delayed transition.
In measuring instruments, mercury thermometers, barometers, and manometers have primarily been replaced by digital sensors, alcohol-based thermometers, or infrared and resistance-based devices. These substitutes offer improved safety, easier calibration, and broader temperature ranges. However, some specialist applications, such as precision meteorological or laboratory measurements, still use mercury due to its stability and accuracy. Even in these areas, regulatory pressure encourages the transition to mercury-free alternatives.
Mercury compounds were once used in catalysts, reagents, and electrode systems in laboratory and industrial chemistry. Many of these functions have been replaced by platinum group metals, ceramic membranes, or organic compounds, though substitution often entails higher costs, altered reactivity, or limited compatibility with legacy systems. The use of mercury in chemical synthesis has declined sharply, especially where closed-loop or green chemistry protocols have been adopted.
In nuclear instrumentation and scientific calibration, mercury is sometimes replaced with cesium, gallium, or tungsten-based systems, depending on density, thermal conductivity, and electrical requirements. However, mercury remains difficult to fully replace in some military, space, and physics applications due to its unique combination of properties, including high density and low vapour pressure.
In artisanal and small-scale gold mining (ASGM), mercury amalgamate fine gold particles, offering a simple but dangerous extraction method. Substitution here is particularly challenging due to economic and technical barriers. Gravity concentration, borax smelting, and cyanide leaching are all viable alternatives, but require training, equipment access, and regulatory support. The success of substitution in ASGM hinges on technical alternatives and broader issues of poverty alleviation, education, and enforcement.
Looking ahead, the global mercury substitution landscape will continue to evolve under the pressure of environmental regulation, ESG criteria, and technological innovation. Substitution will remain as regulatory compliance tightens, and mercury-containing products are phased out of supply chains. Nonetheless, in sectors such as ASGM, scientific research, and strategic technologies, complete elimination of mercury remains difficult, requiring sustained international cooperation, investment in alternatives, and policy mechanisms that address technical and socio-economic barriers.



Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Dr Jenny Watts
Critical Minerals Technologies Expert

Ismet Soyocak
ESG & Critical Minerals Lead

Thomas Shann Mills
Senior Machine Learning Engineer

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

Franklin Avery
Commodity Analyst

Shunjie Zhao (Tony)
Commodity Analyst: APAC

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.