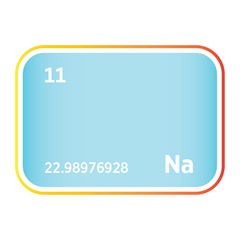
Sodium
Critical Minerals and The Energy Transition
Navigating the Sodium Market
Sodium, a vital element in the industrial and chemical landscape, serves as a broad spectrum of sectors, from manufacturing to energy. As an essential alkali metal, sodium's chemical reactivity and properties are leveraged in various applications, ranging from producing glass and paper to synthesising synthetic rubber and chemicals. Sodium's role in the energy sector is particularly noteworthy, where it acts as a medium in high-temperature molten salt energy storage systems, contributing to the advancement of renewable energy technologies. Moreover, its use in street lighting and as a coolant in some nuclear reactors highlights sodium's versatility and critical role in infrastructure and energy production. The global demand for sodium is propelled by its indispensable uses in water treatment, softening water and purifying brine. However, the sodium market is not without its challenges. The extraction and processing of sodium pose ecological concerns, including habitat disruption and chemical waste management, demonstrating the need for sustainable practices and technological innovations in sodium production and recycling. With sodium supporting industrial processes, ecological responsibility will evolve.
An introduction to sodium
Sodium demand and end-uses
Sodium is a highly reactive alkali metal with extensive industrial, chemical, and technological applications, underpinned by its abundance, low cost, and diverse chemical behaviour. Though rarely used in its pure metallic form, sodium compounds are foundational to modern industry, supporting key sectors such as chemicals manufacturing, metallurgy, energy storage, pharmaceuticals, water treatment, and food production. As demand for alternative battery chemistries and sustainable processes grows, sodium’s strategic relevance steadily increases.
The largest area of sodium demand lies in the chemicals industry, where sodium compounds such as sodium carbonate (soda ash), sodium hydroxide (caustic soda), and sodium sulphate are essential reagents. Sodium carbonate is a cornerstone of glass manufacturing, used in flat glass, bottles, and fibreglass insulation. Caustic soda is widely used in pulp and paper production, alumina refining, textiles, soaps, and detergents, while sodium sulphate is found in powdered detergents and textile processing. These applications form the backbone of bulk chemical production and contribute to sodium’s stable, large-scale industrial consumption.
In metallurgy, sodium is used in specialised alloying and refining processes. Metallic sodium acts as a reducing agent in the production of titanium and zirconium, while sodium vapour is used in specific annealing treatments for improving metal properties. Sodium-based fluxes are also used in aluminium and lead metallurgy, enhancing metal purity and processing efficiency. Although small in volume, these uses are critical in manufacturing high-performance structural materials.
One of the most promising areas of emerging sodium demand is energy storage, where sodium-ion batteries are gaining traction as a viable alternative to lithium-ion systems. Sodium-ion technology benefits from low material costs, wide availability, and improved low-temperature performance, making it attractive for stationary grid storage and energy access in emerging markets. Major battery manufacturers are scaling up sodium-ion production, targeting cost-sensitive and utility-scale applications where lithium supply or pricing constraints are a concern.
Sodium compounds also play a key role in pharmaceuticals and healthcare. Sodium chloride, the most familiar form, is used in intravenous saline solutions, medical rinses, and drug formulation. Sodium bicarbonate is an antacid and buffering agent, while other sodium salts are used in antibiotics, anti-inflammatories, and oral rehydration therapies. The reliable performance and biocompatibility of sodium compounds make them indispensable to clinical and consumer healthcare products.
In environmental and municipal applications, sodium compounds are critical to water treatment and pollution control. Sodium hypochlorite is used as a disinfectant in drinking water and wastewater treatment, while sodium carbonate and caustic soda are used to adjust pH and neutralise acidity in industrial effluents. These functions support regulatory compliance, public health, and industrial environmental performance.
The food industry also accounts for a significant share of sodium compound demand. Sodium chloride, or common salt, is used not only for seasoning but also for preservation and texture modification in processed foods. Sodium-based leavening agents, stabilisers, and emulsifiers are widely used in baking, dairy, and meat products. While excessive sodium intake remains a health concern, its functional importance in food processing ensures continued demand, particularly in developing markets.
Sodium is predominantly sourced from salt deposits and brine sources, with production concentrated in countries such as China, the United States, India, and Germany. The extraction and processing of sodium compounds benefit from well-established infrastructure and relatively low environmental impacts compared to many critical raw materials. However, geopolitical risks, rising energy costs, and growing interest in regional supply resilience are prompting renewed scrutiny of sodium-dependent value chains.
Sodium demand is expected to remain stable in traditional sectors, with notable growth driven by the rise of sodium-ion batteries, particularly for grid-scale storage and lower-cost energy systems. Research into high-performance solid-state sodium batteries, sodium-sulphur flow systems, and hybrid chemistries is ongoing, further positioning sodium as a key enabler of energy access and resilience.
As industries seek abundant, low-cost, and scalable alternatives to constrained materials, sodium stands out for its versatility, functionality, and availability. From glass and chemicals to food and clean energy, sodium’s role in supporting essential systems and next-generation technologies ensures its continued strategic importance in the global economy.
Sodium supply
Sodium supply is abundant, geologically widespread, and largely unconstrained regarding primary availability, processing routes, energy inputs, and regional demand patterns shape its industrial production and downstream applications. Unlike many critical elements, sodium is not typically supply-restricted; it ranks among the most abundant elements in the Earth’s crust and is widely present in sodium chloride (NaCl) in seawater, salt beds, and evaporite deposits.
The primary sources of sodium compounds are halite (rock salt), trona (a sodium carbonate mineral), and synthetic soda ash. Sodium metal is produced mainly through the electrolysis of molten sodium chloride, typically using the Downs cell process, which is energy-intensive and requires specialised infrastructure. The production of sodium hydroxide (caustic soda) and sodium carbonate (soda ash), key industrial sodium compounds, is generally tied to chlor-alkali processes and the Solvay process.
China, the United States, and India are among the top producers of sodium compounds. China dominates global sodium metal output, owing to its expansive chlor-alkali industry and vertical integration into battery and chemical sectors. The United States is a worldwide leader in soda ash production, particularly from natural trona deposits in Wyoming, which offer cost and environmental advantages over synthetic routes. India and several European countries also produce large volumes of caustic soda for domestic chemical industries.
While sodium supply is not geopolitically constrained in the traditional sense, access to refined sodium metal and high-purity sodium compounds can be bottlenecked by energy costs, regulatory compliance, or environmental standards. Sodium metal, in particular, is flammable and reactive, requiring strict handling and transport regulations that limit international trade. Even with widespread geological availability, industrial sodium supply chains remain regionally concentrated and opaque.
Sodium’s strategic relevance is rising, particularly in sodium-ion batteries, which are being developed as a lower-cost, abundant alternative to lithium. As sodium-ion battery technology advances, demand for high-purity sodium salts (such as sodium carbonate and sodium hexafluorophosphate) is expected to increase. This has led several countries to explore domestic supply chain readiness and investment in sodium refining technologies.
Despite the element’s abundance, sodium’s supply dynamics are closely tied to industrial co-production systems, electricity pricing, and technological demand shifts. As the energy transition accelerates, sodium may become more central to battery storage, chemical refining, and next-generation materials, particularly in regions with limited lithium access. In this sense, sodium’s supply chain could become more strategically scrutinised over the next decade.
Sodium substitution
Sodium substitution depends heavily on its application. While sodium is widely used across industries, it is often favoured for cost, abundance, or specific chemical behaviour rather than being technically irreplaceable. In most use cases, alternatives exist, though they may come with performance, safety, cost, or environmental impact trade-offs.
In sodium-ion batteries, which are emerging as a complementary or substitute technology to lithium-ion batteries, lithium is technically superior in energy density, especially for mobile applications. However, sodium offers a cost-effective and more abundant alternative for stationary energy storage, especially where energy density is less critical. If sodium-ion batteries do not scale as anticipated, lithium-based systems or even redox flow batteries could retain dominance in the grid-scale segment. Potassium-ion batteries are also being explored in specific chemistries as a substitute for sodium, offering similar ionic behaviour with potentially different performance profiles.
In the chlor-alkali industry, sodium hydroxide (caustic soda) is a key co-product of chlorine production and is widely used in pulp and paper, textiles, and water treatment. In specific cases, potassium hydroxide can substitute sodium hydroxide, especially in applications requiring higher solubility or reactivity, such as some alkaline batteries and niche chemical synthesis. However, potassium hydroxide is more expensive and less widely available, so substitution is limited to technical requirements.
In glass and ceramics, sodium compounds like sodium carbonate (soda ash) are fluxing agents to lower the melting points. Potassium carbonate or borates can substitute in certain speciality glasses, although they affect final product characteristics such as thermal expansion or transparency. These substitutes are typically reserved for high-end applications like laboratory glassware or display screens.
In chemical synthesis and metallurgy, sodium metal is occasionally used as a reducing agent. Magnesium or calcium may be used as alternatives in these cases, although each presents its own handling and reactivity challenges. For example, magnesium is often a safer or more convenient alternative in organic and inorganic reductions, though it lacks sodium’s exact reaction profile.
In agriculture, sodium nitrate is used only as a fertiliser or preservative. Where required, potassium nitrate or ammonium nitrate are more common substitutes, particularly where plant uptake of potassium or nitrogen is preferable.
While sodium is rarely indispensable, its unique combination of affordability, chemical properties, and global availability means substitutes are usually reserved for specific technical needs or where performance gains justify the cost. As sodium-ion technologies mature and environmental regulations evolve, the calculus around substitution may shift, but sodium's abundance and versatility ensure it will remain central to many industrial systems.



Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Dr Jenny Watts
Critical Minerals Technologies Expert

Ismet Soyocak
ESG & Critical Minerals Lead

Thomas Shann Mills
Senior Machine Learning Engineer

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

Franklin Avery
Commodity Analyst

Shunjie Zhao (Tony)
Commodity Analyst: APAC

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.