Cadmium
Critical Minerals and The Energy Transition
Navigating the Cadmium Market
Cadmium, a toxic yet strategically significant bluish-white metal, is primarily obtained as a by-product of zinc, lead, and copper smelting. Despite its well-documented health and environmental risks, cadmium continues to play a critical role across several industrial and technological domains. Its primary applications span nickel–cadmium (NiCd) batteries, corrosion-resistant coatings, stabilisers for plastics, pigments, and cadmium telluride (CdTe) solar panels, where it remains indispensable to thin-film photovoltaic technology. As the global energy transition accelerates, cadmium’s relevance is being redefined, particularly through its use in CdTe solar modules, which offer cost-effective, high-efficiency alternatives to silicon-based panels. Its presence in rechargeable batteries and semiconductors further links it to sectors prioritising decarbonisation, grid stability, and electrification. However, cadmium’s future remains shaped by mounting regulatory scrutiny, especially in the EU and North America, where restrictions on toxic materials are tightening. This duality between cadmium’s clean energy potential and its environmental liabilities places it at the centre of ongoing materials governance debates. Global supply is relatively small and geographically dispersed, often dependent on the economics of zinc refining. With growing demand in niche applications and limited opportunities for substitution, attention is turning to enhanced recycling pathways, responsible sourcing, and lifecycle management to secure cadmium’s place in a sustainable industrial ecosystem.
An introduction to cadmium
Cadmium demand and end-uses
Cadmium is a soft, bluish-white metal primarily obtained as a by-product of zinc refining, with specialised applications across batteries, coatings, pigments, alloys, and electronics. Though toxic and strictly regulated due to its environmental and health impacts, cadmium remains vital in a narrow range of industrial and technological uses where its properties, such as corrosion resistance, low melting point, and high electrical conductivity, are tricky to substitute. Its role in energy storage and precision components continues to support steady demand despite global efforts to reduce or phase out cadmium in less essential applications.
The most significant and enduring use of cadmium is in nickel–cadmium (NiCd) rechargeable batteries, which are widely used in industrial backup power systems, aviation, railways, emergency lighting, and cordless tools. NiCd batteries offer excellent performance in extreme temperatures, long cycle life, and durability under high-load conditions, making them well suited to demanding environments. While lithium-ion batteries have replaced NiCd in many consumer applications, cadmium-based systems remain preferred in sectors requiring high reliability, long service intervals, or resistance to deep discharge.
Cadmium is also extensively used in corrosion-resistant coatings, particularly in aerospace and defence components, where it is electroplated onto steel and aluminium to protect against marine and atmospheric corrosion. Cadmium coatings offer excellent lubricity, solderability, and resistance to galvanic corrosion—especially important for aircraft fasteners, landing gear, and electrical connectors. Although alternative coatings such as zinc-nickel are being adopted, cadmium remains critical in legacy systems and mission-critical aerospace applications.
In the chemical and pigment industries, cadmium compounds such as cadmium sulphide and cadmium selenide produce bright yellow, orange, and red pigments with high colour stability and resistance to heat and light. These pigments are used in ceramics, plastics, artist paints, and speciality glass, although their use has declined significantly due to environmental regulations and the development of less hazardous alternatives.
Cadmium is used in certain low-melting-point alloys, often in combination with bismuth, lead, or tin, for applications such as fusible plugs, electrical fuses, and safety devices in sprinkler systems. These alloys reliably melt at specific temperatures, triggering safety mechanisms or controlled responses in heat-sensitive systems. Cadmium also contributes to wear resistance and dimensional stability in some precision alloys.
In electronics and optoelectronics, cadmium compounds are used in quantum dots, infrared detectors, and photovoltaic cells. Cadmium telluride (CdTe) is a key material in thin-film solar panels, offering a cost-effective and efficient alternative to crystalline silicon in large-scale solar installations. CdTe solar cells are instrumental in hot, humid, or dusty environments and have been deployed widely in utility-scale projects. While cadmium’s toxicity presents challenges in waste management and recycling, its contribution to clean energy deployment ensures continued relevance in the photovoltaic sector.
Radioactive cadmium-109 is used in scientific and industrial applications, including X-ray fluorescence calibration and radiometric analysis. Cadmium is also used in neutron absorption rods in nuclear reactors, where its high neutron cross-section makes it effective at controlling fission reactions. These specialised nuclear and analytical applications represent small but strategically essential uses of cadmium.
Cadmium’s toxicity has led to strict international regulations on its use, disposal, and recycling. It is classified as a hazardous substance, and many of its former applications, in consumer batteries, jewellery, and household plastics, have been restricted or banned under frameworks such as the EU RoHS directive and the Minamata Convention on Mercury (which includes provisions for heavy metals). As a result, cadmium use today is primarily confined to essential or high-performance applications where alternatives are either technically inferior or economically unviable.
Cadmium demand is expected to remain stable, with modest growth in thin-film photovoltaics and sustained use in NiCd batteries, aerospace coatings, and specialised industrial products. Recycling, particularly from spent batteries and industrial scrap, is increasingly important in securing cadmium supply while reducing environmental risks. Innovations in closed-loop recycling systems and improved waste handling practices are helping mitigate cadmium’s ecological impact.
While cadmium’s hazardous nature limits its broader use, its unique functional properties ensure it remains a critical material in defence, clean energy, and industrial technologies. Its continued relevance depends on responsible sourcing, regulation, and recycling, providing its benefits in essential applications are balanced with a strong commitment to environmental and human health protection.
Cadmium supply
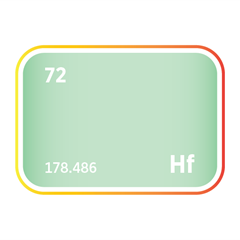
Cadmium occurs almost exclusively as a trace element within zinc ores, particularly sphalerite, the dominant zinc sulphide mineral. In most deposits, cadmium substitutes for zinc in the crystal lattice due to their similar ionic radii, typically ranging from 0.1% to 0.5% cadmium by weight. This geochemical behaviour links cadmium supply directly to the exploitation of zinc-rich geological environments, including sedimentary-exhalative (SEDEX), Mississippi Valley-type (MVT), and volcanogenic massive sulphide (VMS) systems. While greenockite, a rare cadmium sulphide mineral, does occur, it is not mined in its own right due to its extreme rarity. As a result, cadmium's geographic and geological footprint mirrors that of zinc, with no commercially viable primary cadmium deposits currently known.
Cadmium is primarily produced as a by-product of zinc smelting, particularly from the processing of sphalerite ores (zinc sulphide), which often contain trace amounts of cadmium. Because cadmium is not mined directly, its availability is inextricably tied to the global zinc industry. This by-product dependency means that cadmium production is largely unresponsive to direct market demand; instead, it fluctuates based on zinc output, smelting capacity, and metallurgical practices.
Cadmium is typically recovered from zinc refinery residues or dust using pyrometallurgical or hydrometallurgical methods. However, these recovery systems are not universally adopted, especially in regions with stricter environmental regulations or limited economic incentives to extract cadmium. As a result, supply can be both technologically constrained and highly regionalised.
China is the world’s leading cadmium producer, underpinned by its massive zinc refining industry and domestic demand for nickel-cadmium batteries and pigments. Other key producers include South Korea, Japan, Russia, and Kazakhstan, all of which operate substantial base metal smelting infrastructure capable of cadmium recovery. Countries such as Germany, Canada, Mexico, the Netherlands, and the United States also contribute to global supply, often through integrated zinc refining operations.
Additional production is reported from Norway, Bulgaria, Peru, Brazil, Argentina, and North Korea, although volumes from these countries vary and are often tied to local or regional consumption needs. These producers may not compete in large international markets but are significant for niche applications and regional demand, particularly in industrial coatings, stabilisers, and specialised battery chemistries.
Because cadmium supply is governed by zinc production cycles, disruptions in the zinc market, such as mine closures, smelter maintenance, or shifts in zinc pricing, can indirectly tighten cadmium availability. Conversely, surges in zinc output may temporarily increase cadmium volumes, even without corresponding demand growth. This dependency on upstream base metal economics complicates long-term planning for cadmium consumers.
Environmental regulations play a significant role in shaping cadmium supply. In regions with strict emissions controls—particularly across Europe and North America—cadmium recovery is more tightly monitored, and disposal or storage of cadmium-containing residues is regulated under hazardous waste frameworks. In contrast, less regulated jurisdictions may tolerate higher cadmium emissions or fail to recover the metal altogether, which distorts global supply transparency and sustainability efforts.
Recycling contributes only modestly to cadmium supply due to the element's toxic nature and the difficulty of reclaiming it from dispersed consumer goods. However, cadmium is recovered from end-of-life nickel-cadmium batteries in regulated take-back systems, especially in the EU and Japan. Although recycling is not yet a major source, it provides a stable secondary stream that mitigates some reliance on primary production.
Cadmium supply is thus characterised by its secondary status, tight linkage to zinc markets, and exposure to environmental and regulatory pressures. As global attitudes toward toxic metals evolve, particularly in sustainable materials and responsible supply chains, cadmium’s availability may become increasingly dependent on stricter recovery protocols, technological innovation, and substitution in applications where alternatives exist.
Current cadmium producers
Cadmium substitution
Substitution for cadmium is primarily driven by environmental, health, and regulatory pressures due to its toxicity and classification as a hazardous substance in many jurisdictions. While cadmium performs uniquely in specific applications, particularly in electrochemistry, pigments, and coatings, several viable alternatives exist depending on the end-use context. However, each substitute presents performance, cost, or material compatibility trade-offs. The strongest momentum for substitution lies in consumer-facing and environmentally regulated industries. Still, certain high-performance or niche industrial uses rely on cadmium, where alternatives remain technically or economically less viable.
In batteries, cadmium has historically been used in nickel-cadmium (NiCd) rechargeable cells. These have largely been supplanted by nickel-metal hydride (NiMH) and lithium-ion technologies, which offer higher energy densities, longer cycle lives, and fewer environmental concerns. Batteries utilising alternative chemical compositions, notably lithium-ion, have become viable substitutes across most NiCd applications. Regulatory restrictions in the EU and other jurisdictions have accelerated this transition, although NiCd remains used in aviation, emergency power systems, and military platforms.
In coatings and corrosion-resistant alloys, cadmium plating has been valued for its excellent adhesion, sacrificial corrosion protection, and marine durability. However, it is increasingly being replaced by alternatives such as zinc-nickel, tin-zinc, and aluminium-based coatings. For applications where the specific surface characteristics of cadmium are non-critical, such as aircraft fasteners, zinc-nickel coatings present a sustainable and efficient substitute, meeting many aerospace and defence requirements with fewer environmental risks.
In the pigment industry, cadmium sulphide and cadmium selenide have long been used for vivid and thermally stable yellow, orange, and red hues in plastics, ceramics, and glass. While cadmium remains difficult to replace in high-temperature or high-performance applications, eco-friendly pigments such as cerium sulphide and bismuth vanadate are gaining traction. These alternatives are now widely employed in plastics and consumer goods, reducing cadmium-related environmental impacts without compromising colour performance in many use cases.
In PVC stabilisation, cadmium compounds have been widely phased out and replaced by calcium-zinc, barium-zinc, and organotin-based stabilisers. Additionally, barium stabilisers have successfully replaced barium-cadmium stabilisers in flexible PVC production, supporting safer and more sustainable practices in plastics manufacturing, particularly in Europe, where cadmium use is heavily restricted.
In solar photovoltaics, cadmium telluride (CdTe) remains one of the most commercially efficient thin-film technologies, particularly in large-scale and diffuse-light environments. However, research continues into emerging alternatives such as copper indium gallium selenide (CIGS) and perovskite materials, which hold long-term potential to replace CdTe. These technologies are not yet commercially mature but signal a future direction for reducing cadmium use in clean energy infrastructure.
As regulation tightens and materials science progresses, cadmium’s role is expected to decline further, especially in applications where its toxicity outweighs its functional benefits.



Meet the Critical Minerals team
Trusted advice from a dedicated team of experts.

Henk de Hoop
Chief Executive Officer

Beresford Clarke
Managing Director: Technical & Research

Jamie Underwood
Principal Consultant

Dr Jenny Watts
Critical Minerals Technologies Expert

Ismet Soyocak
ESG & Critical Minerals Lead

Thomas Shann Mills
Senior Machine Learning Engineer

Rj Coetzee
Senior Market Analyst: Battery Materials and Technologies

Franklin Avery
Commodity Analyst

Shunjie Zhao (Tony)
Commodity Analyst: APAC

How can we help you?
SFA (Oxford) provides bespoke, independent intelligence on the strategic metal markets, specifically tailored to your needs. To find out more about what we can offer you, please contact us.